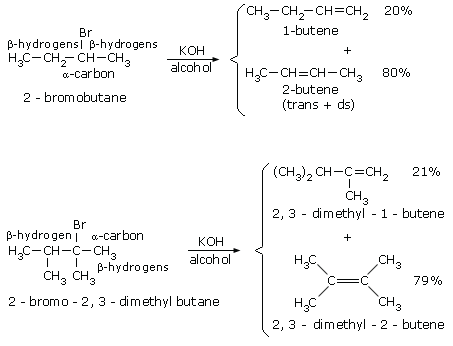![SOLVED: Given the chlorination of acetone shown below, choose the correct rate law: CH3COCH3 + Cl2 -> CH3COCH2Cl + HCl rate = [CH3COCH3] rate = [Cl2] rate = [CH3COCH3][Cl2] rate = [CH3COCH3][Cl2]^(1/2) SOLVED: Given the chlorination of acetone shown below, choose the correct rate law: CH3COCH3 + Cl2 -> CH3COCH2Cl + HCl rate = [CH3COCH3] rate = [Cl2] rate = [CH3COCH3][Cl2] rate = [CH3COCH3][Cl2]^(1/2)](https://cdn.numerade.com/project-universal/previews/ab831c63-ef4a-45e5-af38-e49b2c1cb754.gif)
SOLVED: Given the chlorination of acetone shown below, choose the correct rate law: CH3COCH3 + Cl2 -> CH3COCH2Cl + HCl rate = [CH3COCH3] rate = [Cl2] rate = [CH3COCH3][Cl2] rate = [CH3COCH3][Cl2]^(1/2)

Acetaldehyde and Acetone on reaction with chlorine respectively gives | CLASS 11 | CARBONYL COMP... - YouTube

CXXI.—Preparation of dichlorodinitromethane by the simultaneous nitration and chlorination of acetone - Journal of the Chemical Society, Transactions (RSC Publishing)

Explain why, in the base-catalyzed halogenation of acetone, the second and third halogenations occur on the same carbon as the first, and not on the carbon of the other methyl group.Please explain
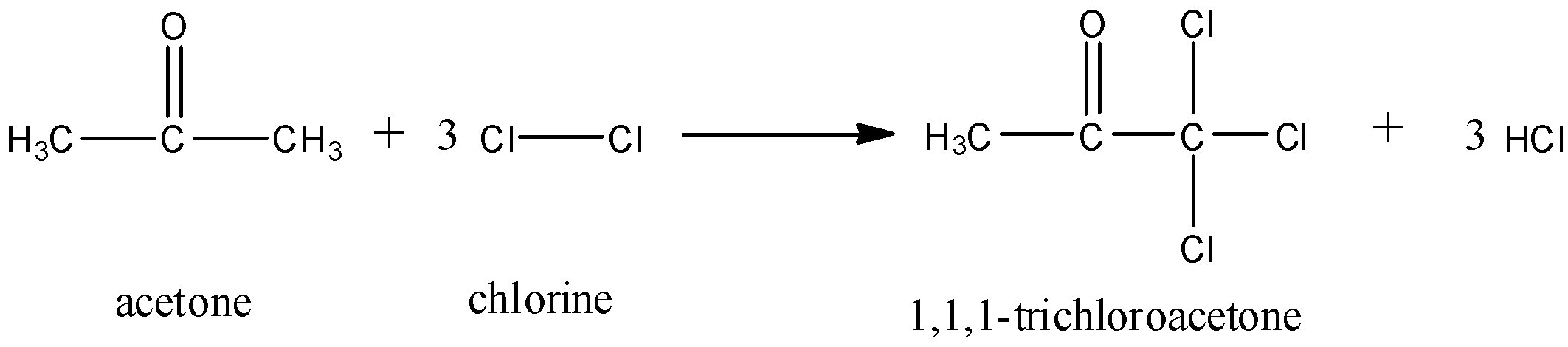
Propan-2-ol on reacting with $C{{l}_{2}}$ produces:A. TrichloroethanolB. TrichloroacetoneC. AcetoneD. None of these

Explain why, in the base-catalyzed halogenation of acetone, the second and third halogenations occur on the same carbon as the first, and not on the carbon of the other methyl group.Please explain




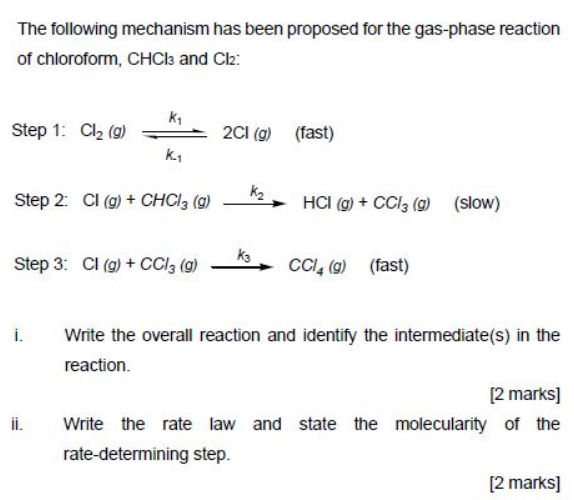

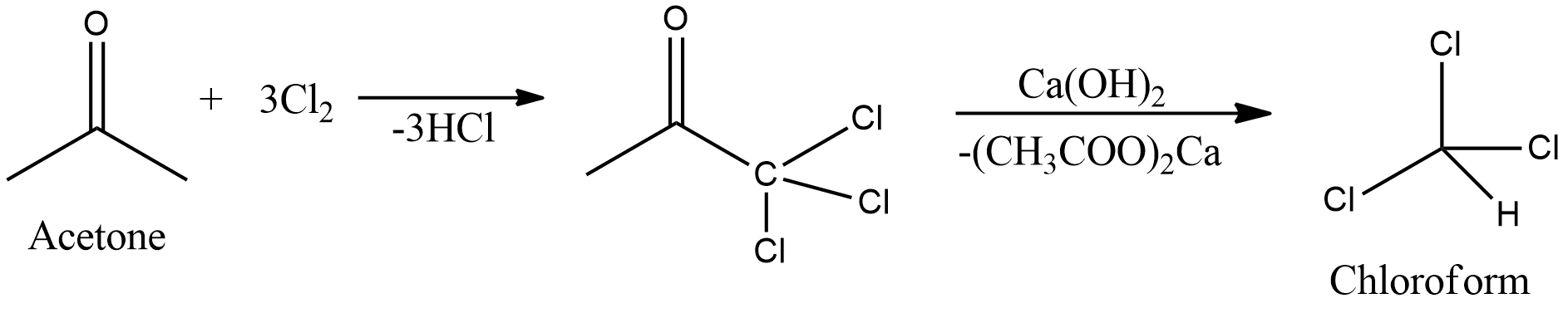
![Telugu] Acetone on reaction with chlorine gives normally Telugu] Acetone on reaction with chlorine gives normally](https://static.doubtnut.com/ss/web-overlay-thumb/6173424.webp)