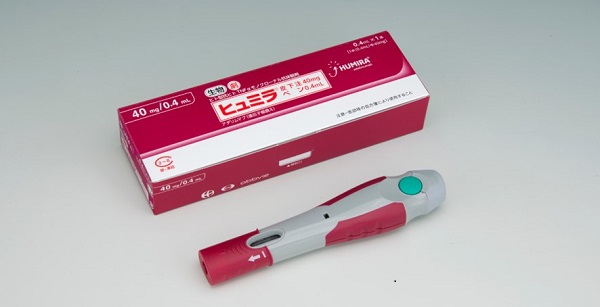
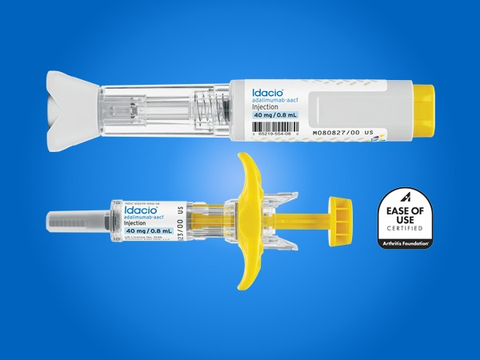
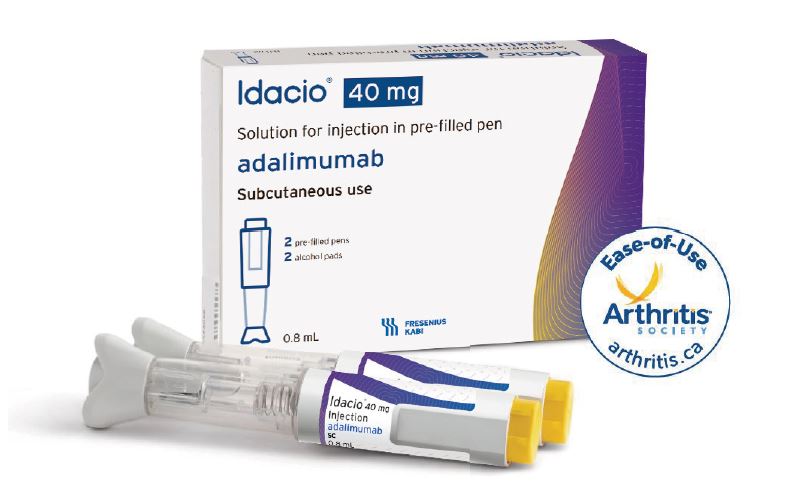
Adalimumab Humira Pen Injection at Rs 9837.5/box | Adalimumab Injections in Ahmedabad | ID: 25436614048

Humira <br> Crohn's Disease Starter Pack <br>Disease-Modifying Antirheumatic Agent Adalimumab, <br>Preservative Free 40 mg / 0.8 mL Subcutaneous <br>Injection Prefilled Auto-Injector Pen 6 Doses<br> Abbott 00074433906
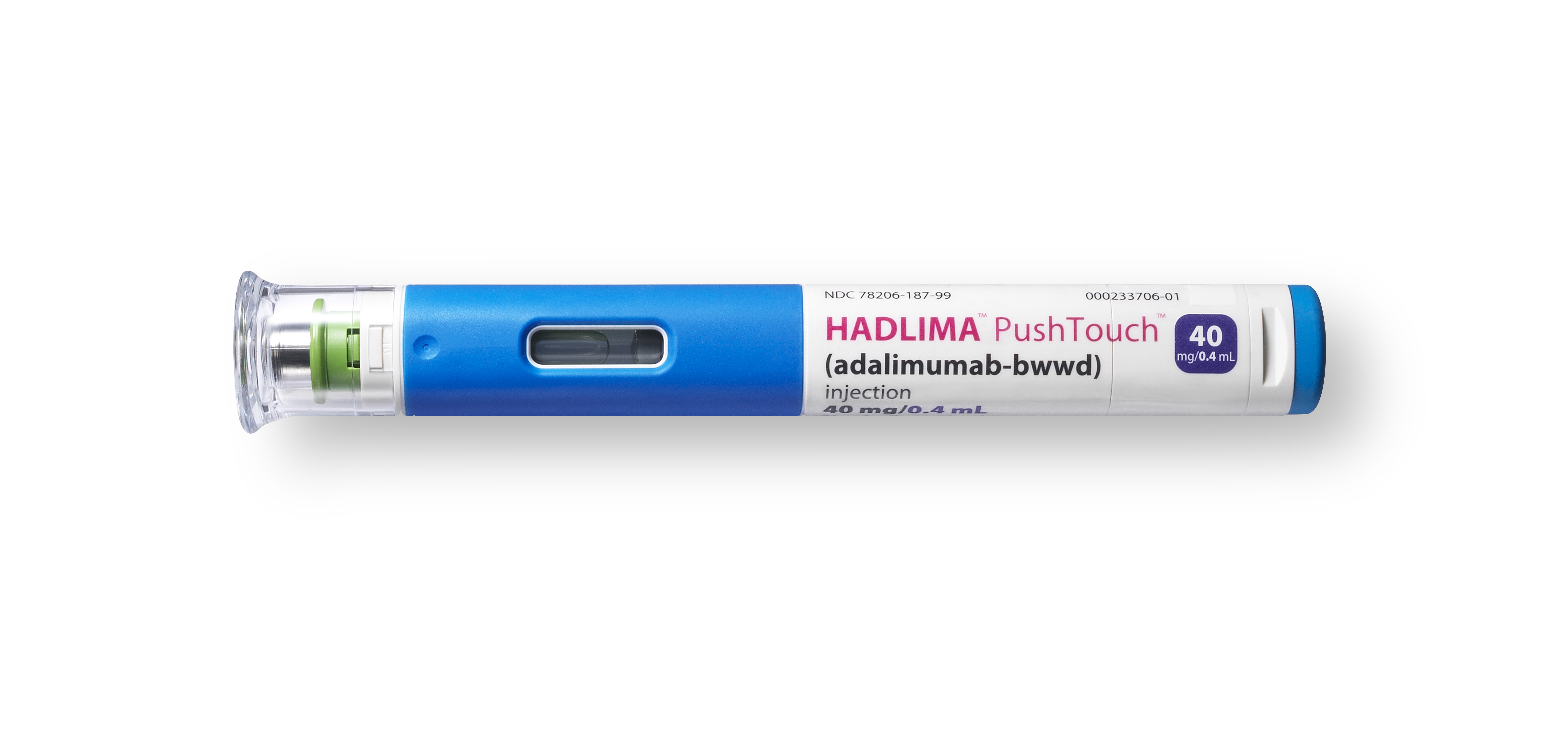
REPEAT/Organon & Samsung Bioepis Announce US Launch of HUMIRA Biosimilar HADLIMA™ (adalimumab-bwwd) in Multiple Presentations Consistent with Originator | Business Wire

These highlights do not include all the information needed to use HUMIRA safely and effectively. See full prescribing information for HUMIRA. HUMIRA ® (adalimumab) injection, for subcutaneous use Initial U.S. Approval: 2002
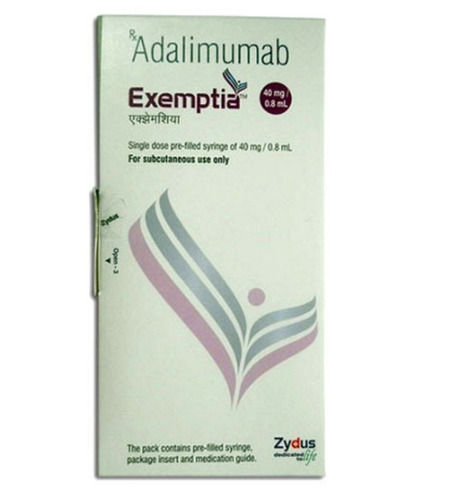
Adalimumab Pre Filled Syringe Injection 40 Mg / 0.8 Ml Dimension(L*W*H): 20 X 5 Feet Foot (Ft) at Best Price in Hyderabad | Healthway Speciality Pharma